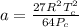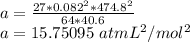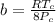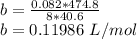Chemistry, 13.02.2020 03:47, tdahna0403
9 The critical temperature and critical pressure of naphthalene are 474.8 K and 40.6 atm, respec- tively. Calculate the van der Waals constants a and b for naphthalene.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2


Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
Do you know the correct answer?
9 The critical temperature and critical pressure of naphthalene are 474.8 K and 40.6 atm, respec- ti...
Questions in other subjects:


Mathematics, 22.10.2019 07:00

Mathematics, 22.10.2019 07:00

Arts, 22.10.2019 07:00



Mathematics, 22.10.2019 07:00



Health, 22.10.2019 07:00