

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
Do you know the correct answer?
A chemist titrates of a hypochlorous acid solution with solution at . Calculate the pH at equivalenc...
Questions in other subjects:

Mathematics, 09.12.2020 22:40




Mathematics, 09.12.2020 22:40



Biology, 09.12.2020 22:40

Mathematics, 09.12.2020 22:40


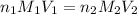
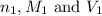 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 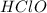
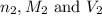 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.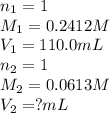
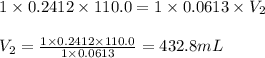
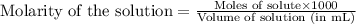 .....(1)
.....(1)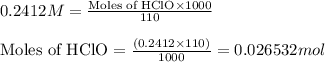
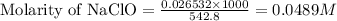
![pH=7+\frac{1}{2}[pK_a+\log C]](/tpl/images/0509/4632/c50ec.png)
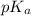 = negative logarithm of weak acid which is hypochlorous acid = 7.50
= negative logarithm of weak acid which is hypochlorous acid = 7.50![pH=7+\frac{1}{2}[7.50+\log (0.0489)]\\\\pH=7+3.09=10.09](/tpl/images/0509/4632/9df6d.png)





