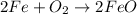A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
with 7.93 g Fe and measure the mass of the final product at 9.50 g.
The balanced equation for the reaction is:
2 Fe + 02 --> 2 FeO
A) The student claims that the percent yield of the reaction was 93.1 %. Support or reject their
claim including a calculation of percent yield as part of your evidence.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3
Do you know the correct answer?
A student reacts steel wool with oxygen by touching the fibers with a 9 volt battery. They begin
Questions in other subjects:




History, 28.08.2019 11:10

Chemistry, 28.08.2019 11:10





Chemistry, 28.08.2019 11:10