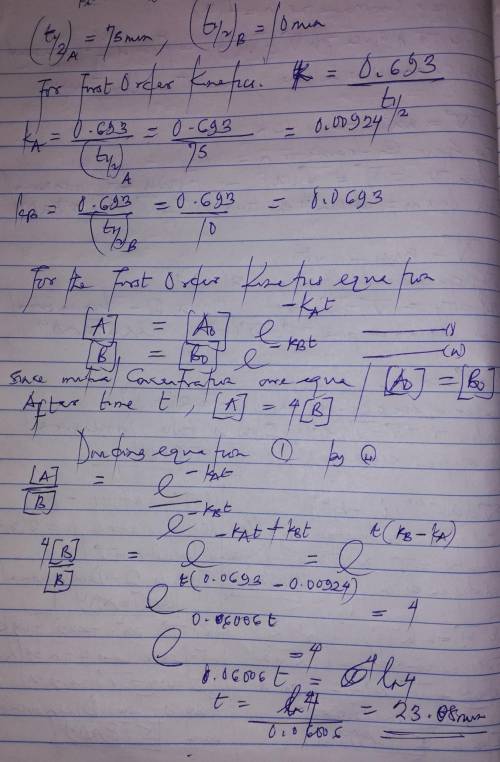Chemistry, 12.02.2020 22:50, sadieruegner393
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. The half-lives are 75.00 min for A and 10.00 min for B. If the concentrations of A and B are equal initially, how long will it take for the concentration of A to be four times that of B

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
Do you know the correct answer?
A flask contains a mixture of compounds A and B. Both compounds decompose by first-order kinetics. T...
Questions in other subjects:


Physics, 18.03.2020 00:52

English, 18.03.2020 00:52




Mathematics, 18.03.2020 00:52



English, 18.03.2020 00:52