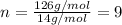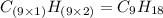Chemistry, 12.02.2020 22:06, hayleneolide
A compound with the empirical formula CH 2 CH2 has a molar mass of 126 126 g/mol. What is the molecular formula for this compound?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Vicky22Shz
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Do you know the correct answer?
A compound with the empirical formula CH 2 CH2 has a molar mass of 126 126 g/mol. What is the molecu...
Questions in other subjects:











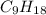
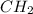
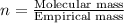
![12+(2\times 1)]=14g/mol](/tpl/images/0508/9817/cd999.png)