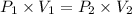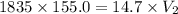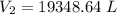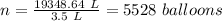Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
Do you know the correct answer?
A 155.0 −L helium tank contains pure helium at a pressure of 1835 psi and a temperature of 298 K. Ho...
Questions in other subjects:

Mathematics, 23.10.2020 07:01


History, 23.10.2020 07:01


Mathematics, 23.10.2020 07:01



Chemistry, 23.10.2020 07:01


Mathematics, 23.10.2020 07:01