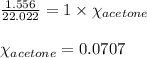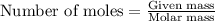Chemistry, 12.02.2020 05:58, mjlchance367
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor pressure of water over the solution is lowered by 1.556 kPa. Given the vapor pressure of water at 65°C is 25.022 kPa, what is the mass of acetone added?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, black99girl
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
Do you know the correct answer?
When a specific amount of acetone (C3H6O) is added to 100.0 g of pure water at 65°C, the vapor press...
Questions in other subjects:

Mathematics, 19.11.2019 07:31

English, 19.11.2019 07:31

History, 19.11.2019 07:31

Social Studies, 19.11.2019 07:31

Mathematics, 19.11.2019 07:31

Mathematics, 19.11.2019 07:31

Mathematics, 19.11.2019 07:31


Chemistry, 19.11.2019 07:31


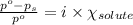
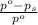 = relative lowering in vapor pressure = 1.556 kPa
= relative lowering in vapor pressure = 1.556 kPa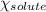 = mole fraction of solute = ?
= mole fraction of solute = ?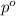 = vapor pressure of pure water = 22.022 kPa
= vapor pressure of pure water = 22.022 kPa