Chemistry, 12.02.2020 05:48, cristykianpour
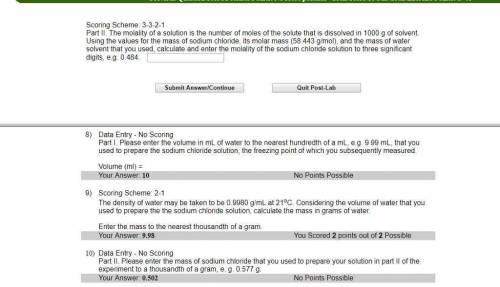
Part II. The molality of a solution is the number of moles of the solute that is dissolved in 1000 g of solvent. Using the values for the mass of sodium chloride, its molar mass (58.443 g/mol), and the mass of water solvent that you used, calculate and enter the molality of the sodium chloride solution to three significant digits, e. g. 0.484.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3


Chemistry, 23.06.2019 11:30, junahsisney
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
Do you know the correct answer?
Part II. The molality of a solution is the number of moles of the solute that is dissolved in 1000 g...
Questions in other subjects:

Mathematics, 30.05.2021 23:40




English, 30.05.2021 23:40

Mathematics, 30.05.2021 23:40

Social Studies, 30.05.2021 23:40


Health, 30.05.2021 23:40

English, 30.05.2021 23:40