
Chemistry, 12.02.2020 05:48, aliveajones2005
A 5.00-mole sample of NH3 gas is kept in a 1.92-L container at 300 K. If the van der Waals equation is assumed for the pressure of the gas, calculate the percent error made in using the ideal-gas equation to calculate the pressure.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1


Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 09:40, 23rwilliamson
Is cutting your nails a physical or chemical change
Answers: 2
Do you know the correct answer?
A 5.00-mole sample of NH3 gas is kept in a 1.92-L container at 300 K. If the van der Waals equation...
Questions in other subjects:


Health, 10.11.2020 19:20

Mathematics, 10.11.2020 19:20

Chemistry, 10.11.2020 19:20



Mathematics, 10.11.2020 19:20


History, 10.11.2020 19:20

Advanced Placement (AP), 10.11.2020 19:20


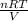
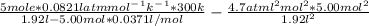
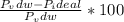 %
%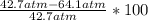 %
%




