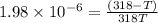Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1
Do you know the correct answer?
A certain liquid has a vapor pressure of 92.0 Torr at 23.0 ∘ C and 378.0 Torr at 45.0∘C.
A. C...
A. C...
Questions in other subjects:

English, 22.03.2021 17:10

Geography, 22.03.2021 17:10

Mathematics, 22.03.2021 17:10

Mathematics, 22.03.2021 17:10

Mathematics, 22.03.2021 17:10


Advanced Placement (AP), 22.03.2021 17:10

Chemistry, 22.03.2021 17:10

Mathematics, 22.03.2021 17:10


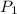 = 92.0 torr,
= 92.0 torr, 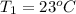 = (23 + 273)K = 296 K
= (23 + 273)K = 296 K
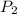 = 378.0 torr,
= 378.0 torr, 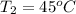 = (45 + 273)K = 318 K
= (45 + 273)K = 318 K
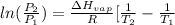
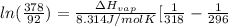
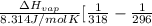
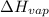 = 2926063.008 J/mol
= 2926063.008 J/mol
![ln (\frac{760 torr}{378 torr}) = -\frac{2926063.008 J/mol }{8.314 J/mol K} [\frac{1}{T} - \frac{1}{318}]](/tpl/images/0508/2864/327ff.png)