
Chemistry, 12.02.2020 05:28, friendsalwaysbae
For a ternary solution at constant T and P, the composition dependence of molar property M is given by: M = x1M1 + x2M2 + x3M3 + x1 x2 x3C where M1, M2, and M3 are the values of M for pure species 1, 2, and 3, and C is a parameter independent of composition. Determine expressions for M¯1,M¯2, and M¯3 by application of Eq. (10.7). As a partial check on your results, verify that they satisfy the summability relation, Eq. (10.11). For this correlating equation, what are the M¯i at infinite dilution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2
Do you know the correct answer?
For a ternary solution at constant T and P, the composition dependence of molar property M is given...
Questions in other subjects:






Biology, 22.11.2019 06:31





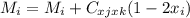 ...1
...1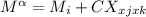 ...2
...2![M_{i} = [\frac{d(nM)}{dn_{i} }]_{P,t,n,j}](/tpl/images/0508/2556/4b4ae.png)




