
Chemistry, 12.02.2020 05:30, pxrnstar4613
Which of the following statements about Avogadro’s Law is false? a. One mole of He occupies 22.4 L at STP. b. At constant P and T, volume and moles of gas are directly proportional. c. At constant T and P, doubling the moles of gas decreases the volume by half. d. At the same T and P, 2 mol of gas occupies twice the volume of one mole of gas. e. An equal number of moles of different gases occupy the same volume at STP.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1


Do you know the correct answer?
Which of the following statements about Avogadro’s Law is false? a. One mole of He occupies 22.4 L a...
Questions in other subjects:


Mathematics, 16.04.2021 20:20



Mathematics, 16.04.2021 20:20





Mathematics, 16.04.2021 20:20


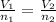 where
where



