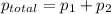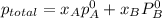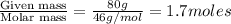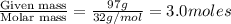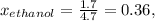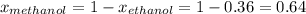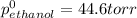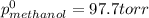Chemistry, 12.02.2020 05:28, usagimiller
At 293 K, methanol has a vapor pressure of 97.7 Torr and ethanol has a vapor pressure of 44.6 Torr. What would be the vapor pressure of a mixture of 80 g of ethanol and 97 g of methanol at 293 K?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2


Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1
Do you know the correct answer?
At 293 K, methanol has a vapor pressure of 97.7 Torr and ethanol has a vapor pressure of 44.6 Torr....
Questions in other subjects:

Advanced Placement (AP), 27.06.2019 11:00

Mathematics, 27.06.2019 11:00

Computers and Technology, 27.06.2019 11:00



Chemistry, 27.06.2019 11:00


Business, 27.06.2019 11:00


Mathematics, 27.06.2019 11:00


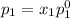 and
and 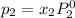
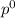 = pressure in the pure state
= pressure in the pure state