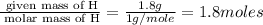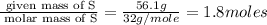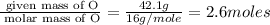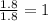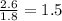A laboratory analysis of a 100 g sample finds it is composed of 1.8 g hydrogen, 56.1 g sulfur, and 42.1 g oxygen. What is its empirical formula? Give your answer in the form H#S#O#, where the number following the element’s symbol corresponds to the subscript in the formula. (Don’t include a 1 subscript explicitly.) For example, the formula CHO would be entered as CH2O.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
Do you know the correct answer?
A laboratory analysis of a 100 g sample finds it is composed of 1.8 g hydrogen, 56.1 g sulfur, and 4...
Questions in other subjects:

Business, 16.03.2020 19:10




English, 16.03.2020 19:11


English, 16.03.2020 19:11