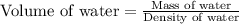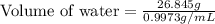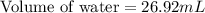Chemistry, 12.02.2020 04:41, carlosgc19
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the total mass to be 32.634g. He then filled the flask with water, weighed again, and obtained a mass of 59.479g. At the temperature of the water, he found that its density was 0.9973 g/mL. a.) What was the mass of the water? (show work)b.) What was the volume of the water? (Show work)c.) What was the volume of the flask? (show work)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2
Do you know the correct answer?
A student obtained a clean, dry, glass-stoppered flask. He weighed the flask stopper and found the t...
Questions in other subjects:


Mathematics, 25.02.2020 18:58


Mathematics, 25.02.2020 18:59

History, 25.02.2020 18:59

Mathematics, 25.02.2020 18:59

Physics, 25.02.2020 18:59

Biology, 25.02.2020 18:59