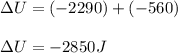Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 23.06.2019 05:30, khaylaperry
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1

Chemistry, 23.06.2019 15:30, 101EXPERIENCE
Which term defines a type of oxygen that forms a protective layer miles above the earth a. fossil fuel b. smog c. pollution d. ozone
Answers: 2

Chemistry, 23.06.2019 19:30, mallorywoods8
(04.03 hc) what type of energy transformation takes place during cellular respiration? use complete sentences to explain how energy is conserved during cellular respiration.
Answers: 1
Do you know the correct answer?
A sample of octane burns releasing 2290 J of heat to the surroundings, and the gases produced expand...
Questions in other subjects:




Physics, 29.03.2021 19:00


Physics, 29.03.2021 19:00

Mathematics, 29.03.2021 19:00




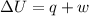
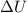 = internal energy
= internal energy