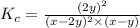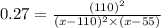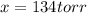Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOC...

Chemistry, 12.02.2020 02:45, ghaithalhamdani
Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOCl(g)
Kp=0.27 at 700 K
A reaction mixture initially contains equal partial pressures of NO and Cl2. At equilibrium, the partial pressure of NOCl was measured to be 110 torr. What were the initial partial pressures of NO and Cl2?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Garciaapril1597
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1


Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Mathematics, 25.05.2021 18:30

Physics, 25.05.2021 18:30



History, 25.05.2021 18:30

Mathematics, 25.05.2021 18:30

English, 25.05.2021 18:30

Mathematics, 25.05.2021 18:30


 and
and 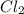 are 134 torr each.
are 134 torr each.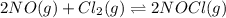
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0507/8534/56950.png)