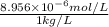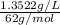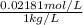Chemistry, 12.02.2020 02:17, ethangorrell67
High concentrations of ammonia (NH3), nitrite ion, and nitrate ion in water can kill fish. Lethal concentrations of these species for rainbow trout are approximately 1.002 mg/L, 0.412 mg/L, and 1352.2 mg/L, respectively. Express these concentrations in molality units, assuming a solution density of 1.00 g/mL. a. m ammoniab. m nitrite ironc. m nitrate ion

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
Do you know the correct answer?
High concentrations of ammonia (NH3), nitrite ion, and nitrate ion in water can kill fish. Lethal co...
Questions in other subjects:



Mathematics, 05.07.2019 15:30

Biology, 05.07.2019 15:30



Mathematics, 05.07.2019 15:30

Mathematics, 05.07.2019 15:30




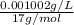
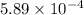 mol/L
mol/L
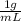 = 1 kg/L
= 1 kg/L
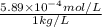
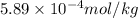
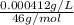
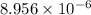 mol/L
mol/L