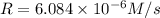The initial rate data at 25 oC are listed for the reaction
NH4+ (aq) + NO2-1 -> N2(g) + H2O(l)
Experiment Initial [NH4+1] Initial [NO2-1] Initial rate (M/s)
1 0.24 0.1 7.2 X10^-6
2 0.12 0.1 3.6 X 10^-6
3 0.12 0.15 5.4 X 10^-6
a. Determine the rate law
b. Determine the value of the rate constant.
c. What is the reaction rate when the concentrations are [NH4+1] = 0.39 M and [NO2-1] = 0.052 M.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 21:00, ciel8809
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1


Chemistry, 23.06.2019 10:00, isaiahromero15
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1
Do you know the correct answer?
The initial rate data at 25 oC are listed for the reaction
NH4+ (aq) + NO2-1 -> N2(g)...
NH4+ (aq) + NO2-1 -> N2(g)...
Questions in other subjects:

English, 06.01.2021 09:50





Mathematics, 06.01.2021 09:50



Business, 06.01.2021 09:50


![=k[NH_4^{+}]^1[NO_2^{-}]^1](/tpl/images/0507/8247/fe0cf.png)
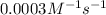
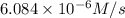 is the reaction rate when the concentrations are
is the reaction rate when the concentrations are ![[NH_4^{+}]](/tpl/images/0507/8247/7aa39.png) = 0.39 M and
= 0.39 M and ![[NO_2^{-}]](/tpl/images/0507/8247/f8e12.png) = 0.052 M.
= 0.052 M.![=k[NH_4^{+}]^x[NO_2^{-}]^y](/tpl/images/0507/8247/8a532.png)
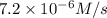
![R=k[0.24 M]^x[0.1 M]^y](/tpl/images/0507/8247/5fc35.png) ...[1]
...[1]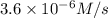
![R'=k[0.12 M]^x[0.1 M]^y](/tpl/images/0507/8247/447c7.png) ...[2]
...[2]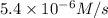
![R''=k[0.12 M]^x[0.15 M]^y](/tpl/images/0507/8247/eedcf.png) ...[3]
...[3]![\frac{R}{R'}=\frac{k[0.24 M]^x[0.1]^y}{k[0.12 M]^x[0.1]^y}](/tpl/images/0507/8247/5b2fa.png)
![\frac{7.2\times 10^{-6} M/s}{3.6\times 10^{-6} M/s}=\frac{k[0.24 M]^x[0.1 M]^y}{k[0.12 M]^x[0.1 M]^y}](/tpl/images/0507/8247/5d06d.png)
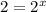
![\frac{R'}{R''}=\frac{k[0.12 M]^x[0.1 M]^y}{k[0.12 M]^x[0.15]^y}](/tpl/images/0507/8247/52502.png)
![\frac{3.6\times 10^{-6} M/s}{5.4\times 10^{-6} M/s}=\frac{k[0.12 M]^x[0.1 M]^y}{k[0.12 M]^x[0.15 M]^y}](/tpl/images/0507/8247/db163.png)
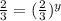
![7.2\times 10^{-6} M/s=k[0.24 M]^1[0.1 M]^1](/tpl/images/0507/8247/29f30.png) ...[1]
...[1]![k=\frac{7.2\times 10^{-6} M/s}{[0.24 M]^1[0.1 M]^1}=0.0003 M^{-1} s^{-1}](/tpl/images/0507/8247/38560.png)
![R=0.0003 M^{-1} s^{-1}\times [0.39 M][0.052 M]](/tpl/images/0507/8247/2f5df.png)