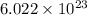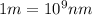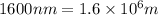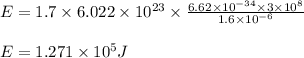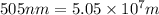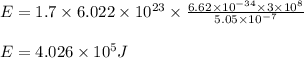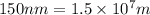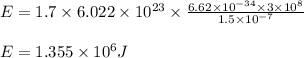Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
Do you know the correct answer?
Determine the energy of 1.70 mol of photons for each of the following kinds of light. (Assume three...
Questions in other subjects:


Mathematics, 07.12.2020 20:00

Physics, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00


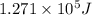

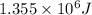
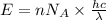 ......(1)
......(1)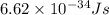
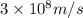
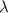 = wavelength of light
= wavelength of light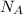 = Avogadro's number =
= Avogadro's number =