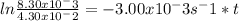The conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °C CH3NC(g)CH3CN(g) is first order in CH3NC with a rate constant of 3.00×10-3 s-1. If the initial concentration of CH3NC is 4.30×10-2 M, the concentration of CH3NC will be 8.30×10-3 M after s have passed.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, princessroyal
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
Do you know the correct answer?
The conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °C CH3NC(g)CH3CN(g) is f...
Questions in other subjects:


Mathematics, 16.05.2021 15:40



Mathematics, 16.05.2021 15:40

Computers and Technology, 16.05.2021 15:40


Mathematics, 16.05.2021 15:40



![ln \frac{[A]_t}{[A]_0} = -kt](/tpl/images/0507/3637/bde68.png)
![[A]_t](/tpl/images/0507/3637/82372.png) is the concentration of the reactant in the time t,
is the concentration of the reactant in the time t, ![[A]_0](/tpl/images/0507/3637/7075c.png) is the initial concentration of the reactant, k is rate constant and t is time. Replacing with values of the problem:
is the initial concentration of the reactant, k is rate constant and t is time. Replacing with values of the problem: