Chemistry, 10.02.2020 23:04, natalie2sheffield
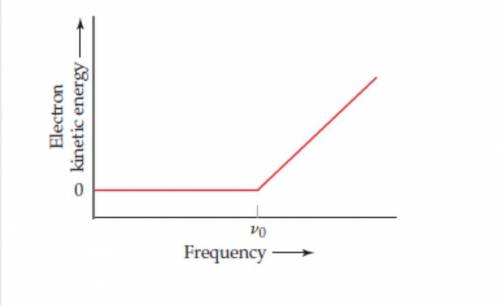
In an experiment to study the photoelectric effect, a scientist measures the kinetic energy of ejected electrons as afunction of the frequency of radiation hitting a metal surface. She obtains the following plot The point labeled " v0 "corresponds to light with a wavelength of 680 nrn (a)What is the value of in 5-1? (b)What is the value of the work functionof the metal in units of of ki/mol ejected electrons? (c) What happens when the metal is irradiated with light of frequencyless than Vo? (d) Note that when the frequency of the light is greater than Vo, the plot shows a straight line with a nonzeroslope. Why is this the case? (e) Can you determine the slope of the line segment discussed in part (d)? Explain.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2


Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1
Do you know the correct answer?
In an experiment to study the photoelectric effect, a scientist measures the kinetic energy of eject...
Questions in other subjects:


Mathematics, 29.08.2021 22:30



Mathematics, 29.08.2021 22:30

Business, 29.08.2021 22:30

History, 29.08.2021 22:30

Social Studies, 29.08.2021 22:30

Mathematics, 29.08.2021 22:30

History, 29.08.2021 22:30