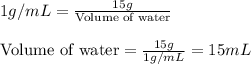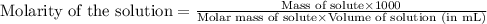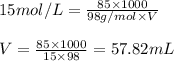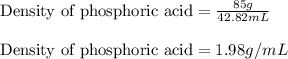Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
Do you know the correct answer?
Concentrated phosphoric acid is sold as a solution of 85% phosphoric acid and 15% water by mass. Giv...
Questions in other subjects:

Mathematics, 12.02.2021 17:20

Social Studies, 12.02.2021 17:20

Mathematics, 12.02.2021 17:20



Mathematics, 12.02.2021 17:20


English, 12.02.2021 17:20



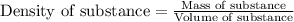 ......(1)
......(1)