The decomposition of formic acid follows first-order kinetics. HCO2H(g) → CO2(g) + H2(g) The half-life for the reaction at 550°C is 24 seconds. How many seconds does it take for the formic acid concentration to decrease by 87.5%? (1) 4.6 seconds (2) 36 seconds (3) 48 seconds (4) 72 seconds (5) 96 seconds

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
Do you know the correct answer?
The decomposition of formic acid follows first-order kinetics. HCO2H(g) → CO2(g) + H2(g) The half-li...
Questions in other subjects:


Mathematics, 29.01.2021 18:00

History, 29.01.2021 18:00





Mathematics, 29.01.2021 18:00

Social Studies, 29.01.2021 18:00

Mathematics, 29.01.2021 18:00


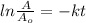
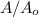 will become 0.125
will become 0.125