
Chemistry, 10.02.2020 21:27, corey36dylon
What is the boiling point of a solution made by dissolving 23.6 g of a non-ionizing solute with a molar mass of 103.5 g/mol in 188 g of water?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 10:20, Thejollyhellhound20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
Do you know the correct answer?
What is the boiling point of a solution made by dissolving 23.6 g of a non-ionizing solute with a mo...
Questions in other subjects:

Chemistry, 21.02.2021 18:20

Geography, 21.02.2021 18:20



Mathematics, 21.02.2021 18:20



English, 21.02.2021 18:20

Social Studies, 21.02.2021 18:20





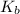 = molal boiling point elevation constant = 0.512°C/m.g
= molal boiling point elevation constant = 0.512°C/m.g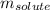 = Given mass of solute = 23.6 g
= Given mass of solute = 23.6 g = Molar mass of solute = 103.5 g/mol
= Molar mass of solute = 103.5 g/mol = Mass of solvent (water) = 188 g
= Mass of solvent (water) = 188 g





