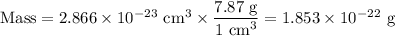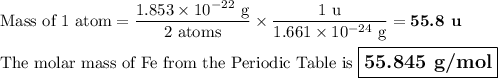Metallic iron has a body-centered cubic lattice with all atoms at lattice points and a unit cell whose edge length is 286.6 pm. the density of iron is 7.87 g cm–3 . what is the mass of an iron atom? compare this value with the value you obtain from the molar mass

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3


Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
Do you know the correct answer?
Metallic iron has a body-centered cubic lattice with all atoms at lattice points and a unit cell who...
Questions in other subjects:

History, 14.12.2020 23:10


Mathematics, 14.12.2020 23:10

History, 14.12.2020 23:10




Mathematics, 14.12.2020 23:10