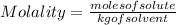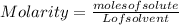The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = k...

The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = kfm
where kf is the freezing point depression constant and m is the molality of the solution. which of the statements explains why molality is used instead of molarity in this equation?
a. molality does not appear in many equations, so it is used here to distinguish this equation from other similar ones.
b. as the temperature of a solution changes, its volume will also change, which will affect its molarity but not its molality.
c. in solutions, moles are not directly related to grams and the freezing point of a solution is dependent solely on the number of grams of solute.
d. the equation was originally published with m as a typo, rather than m, but the values are close enough that the equation is still valid.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
Do you know the correct answer?
Questions in other subjects:




Mathematics, 08.01.2021 18:20


Mathematics, 08.01.2021 18:20

Biology, 08.01.2021 18:20

Business, 08.01.2021 18:20