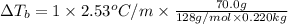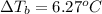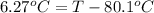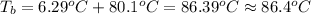Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a nonvolatile nonelectrolyte), in 220.0 g of benzene, c6h6. the kb for benzene = 2.53oc/m. the boiling point of pure benzene is 80.1oc.. ans: 86.4 degrees celsius. i did this so far. 1)70g c10h8(1mol c10h8/128gc10h8)= .546mol c10h8. benzene)=2.482m. 3) (2.482)(2.53 c/m)=6.289. i'm not sure if i am starting this off right, can anyone me get the correct answer? ans ty!

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 22:30, creepycrepes
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
Do you know the correct answer?
Calculate the boiling point of a solution prepared by dissolving 70.0 g of naphthalene, c10h8 (a non...
Questions in other subjects:




Mathematics, 23.02.2020 19:34

Mathematics, 23.02.2020 19:34

Mathematics, 23.02.2020 19:34

Mathematics, 23.02.2020 19:34




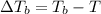
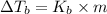
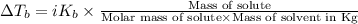
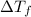 =Elevation in boiling point
=Elevation in boiling point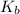 = Boiling point constant of solvent = 2.53 °C/m(benzene)
= Boiling point constant of solvent = 2.53 °C/m(benzene)