
Chemistry, 29.01.2020 05:46, Ezekielcassese
Acertain liquid x has a normal boiling point of 118.90 °c and a boiling point elevation constant kb = 0.82 °c*kg*mol^-1. calculate the boiling point of a solution made of 54.g of potassium bromide (kbr) dissolved in 750. g of x. be sure your answer is rounded to the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, cathydaves
What is the chemical formula of the following compound
Answers: 1

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Do you know the correct answer?
Acertain liquid x has a normal boiling point of 118.90 °c and a boiling point elevation constant kb...
Questions in other subjects:

Advanced Placement (AP), 01.06.2020 20:58

Mathematics, 01.06.2020 20:58




History, 01.06.2020 20:59

French, 01.06.2020 20:59

Health, 01.06.2020 20:59

History, 01.06.2020 20:59






 = molal boiling point elevation constant = 0.82°C/m
= molal boiling point elevation constant = 0.82°C/m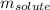 = Given mass of solute (potassium bromide) = 54. g
= Given mass of solute (potassium bromide) = 54. g = Molar mass of solute (potassium bromide) = 119 g/mol
= Molar mass of solute (potassium bromide) = 119 g/mol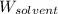 = Mass of solvent (liquid X) = 750. g
= Mass of solvent (liquid X) = 750. g




