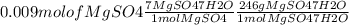Chemistry, 29.01.2020 04:50, taylabrown2013
On the first day of your new job as a chemist, you are given a bottle of magnesium sulfate and asked to make 30 ml of 0.3 m mgso4. the formula on the bottle is mgso4∗7h2o (also known as epsom salt). calculate the amount of salt you need (in milligrams).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
Do you know the correct answer?
On the first day of your new job as a chemist, you are given a bottle of magnesium sulfate and asked...
Questions in other subjects:

History, 10.12.2020 04:50






Chemistry, 10.12.2020 04:50


Mathematics, 10.12.2020 04:50

Health, 10.12.2020 04:50