
Chemistry, 29.01.2020 02:45, gizmo50245
Asample of 1.000 g of a compound containing carbon and hydrogen reacts with oxygen at elevated temperature to yield 0.692 g h₂o and 3.381 g co₂. (a) calculate the masses of c and h in the sample. (b) does the compound contain any other elements

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:40, ricardoamora54
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1


Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
Do you know the correct answer?
Asample of 1.000 g of a compound containing carbon and hydrogen reacts with oxygen at elevated tempe...
Questions in other subjects:











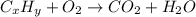
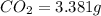
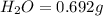
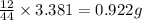 of carbon will be contained.
of carbon will be contained.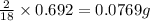 of hydrogen will be contained.
of hydrogen will be contained.



