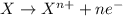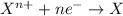Chemistry, 29.01.2020 02:52, katelynamber10
Acalled a reductant or reducer) is an element or compound that loses (or "donates") an electron to an electron recipient (oxidizing agent) in a redox chemical reaction.
a reducing agent is thus oxidized when it loses electrons in the redox reaction. reducing agents "reduce" (or, are "oxidized" by) oxidizing agents. oxidizers "oxidize" (that is, are reduced by) reducers.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1


Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
Do you know the correct answer?
Acalled a reductant or reducer) is an element or compound that loses (or "donates") an electron to a...
Questions in other subjects:








Mathematics, 26.06.2020 15:01