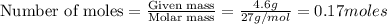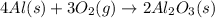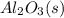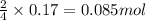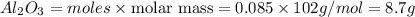Chemistry, 29.01.2020 00:42, melissareid65
Luminum and oxygen react according to the following equation: 4al(s) +3o2(g) --> 2al2o3(s) what mass of al2o3, in grams, can be made by reacting 4.6 g al with excess oxygen?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1


Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
Do you know the correct answer?
Luminum and oxygen react according to the following equation: 4al(s) +3o2(g) --> 2al2o3(s) what...
Questions in other subjects:


Mathematics, 26.07.2021 18:20

Mathematics, 26.07.2021 18:20




Mathematics, 26.07.2021 18:20


History, 26.07.2021 18:20


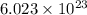 of particles.
of particles.