
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 m⁻¹s⁻¹ : > 2so₃g + 2so₂g o₂g suppose a vessel contains so₃ at a concentration of 1.44m . calculate the concentration of so₃ in the vessel 0.240 seconds later. you may assume no other reaction is important. round your answer to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, lexybellx3
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3


Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
Do you know the correct answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 m⁻...
Questions in other subjects:

Mathematics, 02.07.2019 10:40


Biology, 02.07.2019 10:40

Mathematics, 02.07.2019 10:40

Social Studies, 02.07.2019 10:40




History, 02.07.2019 10:40


![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0479/1273/ccade.png)
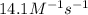
![[A_t]](/tpl/images/0479/1273/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0479/1273/dc622.png) = initial concentration = 1.44 M
= initial concentration = 1.44 M![14.1\times 0.240=\frac{1}{[A_t]}-\frac{1}{1.44}](/tpl/images/0479/1273/20832.png)
![[A_]t=0.24M](/tpl/images/0479/1273/ae6b5.png)




