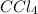Chemistry, 28.01.2020 20:47, cardenas08
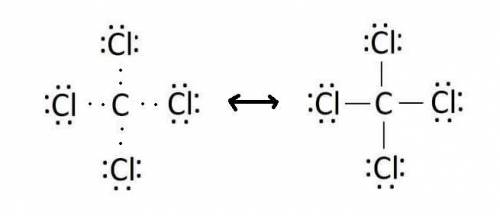
Use lewis theory to draw the structure of the compound containing 1 carbon and 4 chlorine atoms. what would you find surrounding the central atom of this ion?
a. 1 double bond.
b. no double bonds.
c. 2 double bonds.
d. 1 triple bond.
e. none of the above

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
Do you know the correct answer?
Use lewis theory to draw the structure of the compound containing 1 carbon and 4 chlorine atoms. wha...
Questions in other subjects:



Health, 13.09.2019 22:30



Biology, 13.09.2019 22:30