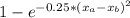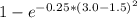Chemistry, 28.01.2020 20:48, Tabbicat021
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding. the electronegativities for and are 1.5 and 3.0 respectively. calculate the fraction of the bonding that is ionic. (enter your answer to three significant figures.) =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 06:10, ridzrana02
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
Do you know the correct answer?
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding...
Questions in other subjects:

Social Studies, 19.07.2019 13:00

Biology, 19.07.2019 13:00

Social Studies, 19.07.2019 13:00




Biology, 19.07.2019 13:00