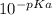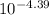Chemistry, 28.01.2020 18:51, nmartin5185
In an aqueous solution of a certain acid the acid is 4.4% dissociated and the ph is 3.03. calculate the acid dissociation constant ka of the acid. round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
Do you know the correct answer?
In an aqueous solution of a certain acid the acid is 4.4% dissociated and the ph is 3.03. calculate...
Questions in other subjects:

History, 09.04.2020 06:29

History, 09.04.2020 06:29


History, 09.04.2020 06:29

English, 09.04.2020 06:29


Chemistry, 09.04.2020 06:29


Mathematics, 09.04.2020 06:29