Chemistry, 28.01.2020 07:31, hollybell1213
The average mass of a carbon atom is 12.011. assuming you were able to pick up only one carbon atom, the chance that you would randomly get one with a mass of 12.011 is a. 0%. b. 0.011%. c. about 12%. d. 12.011%. e. greater than 50%. f. none of these is true. explain.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
Do you know the correct answer?
The average mass of a carbon atom is 12.011. assuming you were able to pick up only one carbon atom,...
Questions in other subjects:

Biology, 12.10.2021 05:00

Biology, 12.10.2021 05:10

Biology, 12.10.2021 05:10

Mathematics, 12.10.2021 05:10



Social Studies, 12.10.2021 05:10



Mathematics, 12.10.2021 05:10


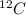 there is 99%
there is 99% 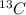 is present. Hence, its mass will be calculated as follows.
is present. Hence, its mass will be calculated as follows.