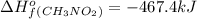Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced combustion equation: 2ch3no2(l)+3/2o2(g)→2co2(g)+3h2o(g) +n2(g). the standard enthalpy of combustion for nitromethane is −709.2kj/mol. calculate the standard enthalpy of formation(delta h formation) for nitro-methane.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
Do you know the correct answer?
Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced com...
Questions in other subjects:


Social Studies, 05.05.2021 19:20



Mathematics, 05.05.2021 19:20

Physics, 05.05.2021 19:20




Mathematics, 05.05.2021 19:20


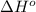
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0475/2966/45485.png)
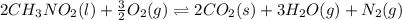
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})+(n_{(N_2)}\times \Delta H^o_f_{(N_2)})]-[(n_{(CH_3NO_2)}\times \Delta H^o_f_{(CH_3NO_2)})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0475/2966/ea72d.png)
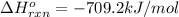
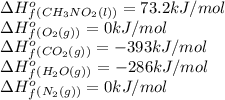
![-709.2=[(2\times -393)+(3\times -286)+(1\times 0)]-[(2\times \Delta H^o_f_{(CH_3NO_2)})+(\frac{3}{2}\times 0)]](/tpl/images/0475/2966/4d16f.png)