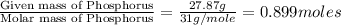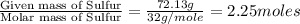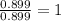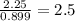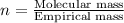Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1


Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
Do you know the correct answer?
Ferrophosphorus (fe2p) reacts with pyrite (fes2), producing iron (ii) sulfide and a compound that is...
Questions in other subjects:

French, 06.05.2020 08:07



Mathematics, 06.05.2020 08:07

Mathematics, 06.05.2020 08:07


Mathematics, 06.05.2020 08:07




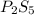 and
and 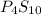 respectively.
respectively.