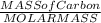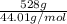Chemistry, 28.08.2019 01:00, ashleyremon
The molar mass of carbon dioxide (co2) is 44.01 g/mol. the molar mass of water (h2o) is 18.01 g/mol. a reaction uses 528 g of co2. how many moles of water are used in this reaction?

Answers: 1
Similar questions


Biology, 20.07.2019 17:00, davidmfox5890
Answers: 2

Do you know the correct answer?
The molar mass of carbon dioxide (co2) is 44.01 g/mol. the molar mass of water (h2o) is 18.01 g/mol....
Questions in other subjects:

Mathematics, 29.06.2019 16:00


Chemistry, 29.06.2019 16:10


Social Studies, 29.06.2019 16:10

Social Studies, 29.06.2019 16:10


Health, 29.06.2019 16:10


World Languages, 29.06.2019 16:10