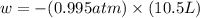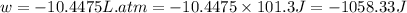Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
Do you know the correct answer?
Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constan...
Questions in other subjects:

Mathematics, 23.09.2020 16:01

Mathematics, 23.09.2020 16:01

Mathematics, 23.09.2020 16:01




Biology, 23.09.2020 16:01

History, 23.09.2020 16:01

Computers and Technology, 23.09.2020 16:01

Mathematics, 23.09.2020 16:01


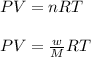
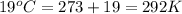
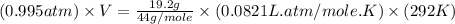
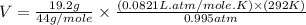
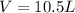
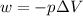
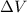 = volume = 10.5 L
= volume = 10.5 L