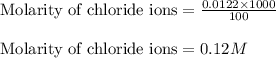Suppose of zinc chloride is dissolved in of a aqueous solution of ammonium sulfate. calculate the final molarity of zinc cation in the solution. you can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1


Chemistry, 23.06.2019 03:30, jennelledenise
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
Do you know the correct answer?
Suppose of zinc chloride is dissolved in of a aqueous solution of ammonium sulfate. calculate the fi...
Questions in other subjects:

Mathematics, 17.12.2019 09:31

Social Studies, 17.12.2019 09:31

Social Studies, 17.12.2019 09:31

History, 17.12.2019 09:31



Mathematics, 17.12.2019 09:31



Mathematics, 17.12.2019 09:31


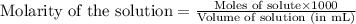 .....(1)
.....(1)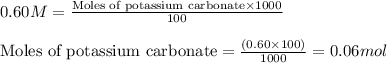
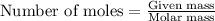
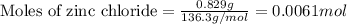
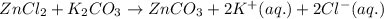
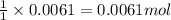 of potassium carbonate
of potassium carbonate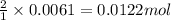 of acetate ion
of acetate ion