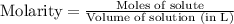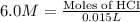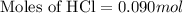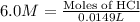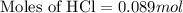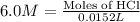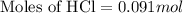Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
Do you know the correct answer?
Part iv. for each trial, calculate the number moles of 6.0 m hcl used in the reaction. report your a...
Questions in other subjects:




Mathematics, 07.05.2020 01:05

Mathematics, 07.05.2020 01:05



Physics, 07.05.2020 01:05


Spanish, 07.05.2020 01:05