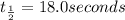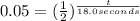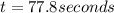N2o5(g) → no3(g) + no2(g)
this reaction is 1st order with half-life = 18.0 seconds. how long w...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Biology, 11.02.2021 05:10


Mathematics, 11.02.2021 05:10

Mathematics, 11.02.2021 05:10





Mathematics, 11.02.2021 05:10

English, 11.02.2021 05:10


![[N_{2}O_{5}]=[N_{2}O_{5}]_{0}\times (\frac{1}{2})^{\frac{t}{t_{\frac{1}{2}}}}](/tpl/images/0470/3124/25a61.png)
![[N_{2}O_{5}]](/tpl/images/0470/3124/8f3d9.png) is the concentration of
is the concentration of 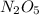 after "t" time,
after "t" time, ![[N_{2}O_{5}]_{0}](/tpl/images/0470/3124/64fd1.png) is the initial concentration of
is the initial concentration of 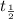 is half life
is half life![\frac{[N_{2}O_{5}]}{[N_{2}O_{5}]_{0}}=\frac{100-95}{100}=0.05](/tpl/images/0470/3124/004e3.png) and
and