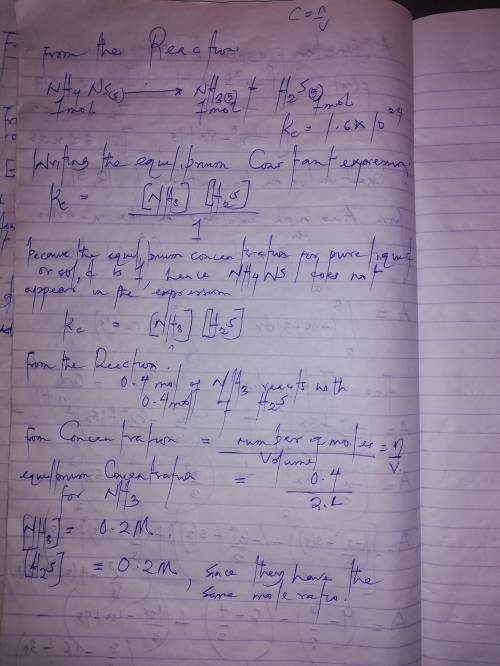Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, yselahernandez02
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1

Chemistry, 23.06.2019 06:00, mustafajibawi1
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 10:00, kidre96521
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
Do you know the correct answer?
When solid nh₄hs and 0.400 mol nh₃(g) were placed in a vessel of volume 2.0 l at 24°c, the equilibri...
Questions in other subjects:





Mathematics, 11.04.2020 05:13


Mathematics, 11.04.2020 05:13

Biology, 11.04.2020 05:13