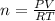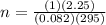Chemistry, 25.01.2020 02:31, carbpotatoes
Yeast and other organisms can convert glucose (c6h12o6) to ethanol (ch3ch2oh) through a process called alcoholic fermentation. the net reaction is:
(l) +2co2(g)
calculate the mass of glucose required to produce 2.25l of co2 measured at p=1 atm and t=295k

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Do you know the correct answer?
Yeast and other organisms can convert glucose (c6h12o6) to ethanol (ch3ch2oh) through a process call...
Questions in other subjects:



Biology, 27.12.2019 20:31


Computers and Technology, 27.12.2019 20:31

Mathematics, 27.12.2019 20:31



Mathematics, 27.12.2019 20:31