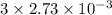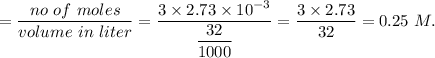Chemistry, 25.01.2020 02:31, Ayyyyeeeeeeewuzgud
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivalence point is reached when 24.83 ml of naoh solution is added. what is the concentration of the unknown h3po4 solution? the neutralization reaction is
h3po4(aq)+3naoh(aq)→3h2o(l)+na3po4( aq)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1
Do you know the correct answer?
A32.00 ml sample of an unknown h3po4 solution is titrated with a 0.110 m naoh solution. the equivale...
Questions in other subjects:

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10

Mathematics, 20.01.2021 01:10






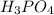 in sample is 0.25 M.
in sample is 0.25 M.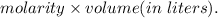
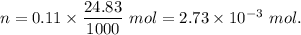
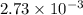 mol of NaOH reacts with
mol of NaOH reacts with