
Chemistry, 25.01.2020 01:31, princeofpowerjr
Awater treatment plant receives the source water with an average ca2+ concentration of 46.9 mg/l and mg2+ concentration of 14.8 mg/l. the plant is treating 80 million gallons of water per day. what mass of solids will be produced per day if all of the calcium and magnesium are converted to caco3(s) and mg(oh)2(s) in the softening process? give your answer in kg.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2


Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
Do you know the correct answer?
Awater treatment plant receives the source water with an average ca2+ concentration of 46.9 mg/l and...
Questions in other subjects:

Biology, 26.08.2020 14:01

Geography, 26.08.2020 14:01


Geography, 26.08.2020 14:01



Health, 26.08.2020 14:01




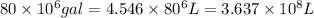




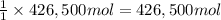 of calcium carbonate
of calcium carbonate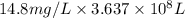

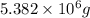

 of magnesium hydroxide
of magnesium hydroxide




