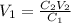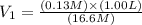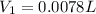Chemistry, 24.01.2020 21:31, RoyalGurl01
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many milliliters of the stock solution do you require to make up 1.00 l of 0.13 m hno3?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 23:00, soccerplayer17
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
Do you know the correct answer?
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many millil...
Questions in other subjects:

Mathematics, 31.03.2021 06:20




English, 31.03.2021 06:20



Mathematics, 31.03.2021 06:20

Physics, 31.03.2021 06:20



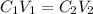
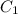 and
and 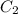 are initial and final concentration respectively.
are initial and final concentration respectively. 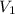 and
and  are initial and final volume respectively.
are initial and final volume respectively.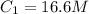 ,
,  and
and